WHO accelerates testing for mpox.

TEXT : Samara Muehitari
At the end of August 2024, the WHO requested manufacturers to submit their products for review to the 'Emergency Use Listing' to expedite access to mpox testing kits. This push for accelerated testing follows the declaration of a public health emergency concerning mpox announced on August 14. The outbreak in 2022 resulted in many casualties, highlighting the need for swift responses in the future. In this second pandemic, infections of a highly lethal new strain have also been reported worldwide. I would like to report on the current status of mpox measures.
What the WHO aims to achieve with this request is a rapid response for pandemic control. Pharmaceutical companies are applying for emergency use authorization of their vaccines on the "Emergency Use List," and the WHO is conducting expedited reviews. The goal is to make it easier for countries struggling with the spread of mpox to procure necessary medical products. Mpox infections surged in 2022, leading the WHO to declare a state of emergency in July. Although the spread of infections subsided by May 2023, nine months after the declaration, over 87,000 cases and 140 deaths were ultimately reported from 111 countries. WHO Director-General Tedros Adhanom said at a press conference, "Mpox will continue to pose significant public health challenges, and proactive measures are necessary.

On August 14, 2024, the WHO declared a "Public Health Emergency of International Concern" in response to the spread of mpox infections from Congo to neighboring countries. The WHO analyzed the emergence of new variants and the reports of infections in neighboring countries where outbreaks had not been previously reported as concerning developments. This declaration came less than a year after the previous outbreak was contained. Director-General Tedros Adhanom stated at a press conference, "There is a possibility of the spread of infections both within and outside Africa, and an international coordinated response is necessary.
Mpox is transmitted through close contact. Typically, it progresses with mild symptoms, but there are rare cases of death. Initially, the spread of infections was observed within Congo, where mpox has been endemic for many years. Subsequently, the infection expanded to refugee camps with high population density and poor sanitary conditions. The outbreaks in these camps, which have high levels of human movement, accelerated the speed of the pandemic. Additionally, the emergence of new variants has further worsened the situation. The variant "Clade 1b" is said to have a higher fatality rate than the original strain, with an estimated 4 out of 100 people dying. The infection that started in Congo has spread to neighboring countries such as Kenya, Rwanda, and Uganda, leading the WHO to declare a state of emergency. Subsequently, cases have also been confirmed in distant locations like Sweden and Thailand. In Africa, where the outbreak continues, over 500 people have died this year, and the spread persists.
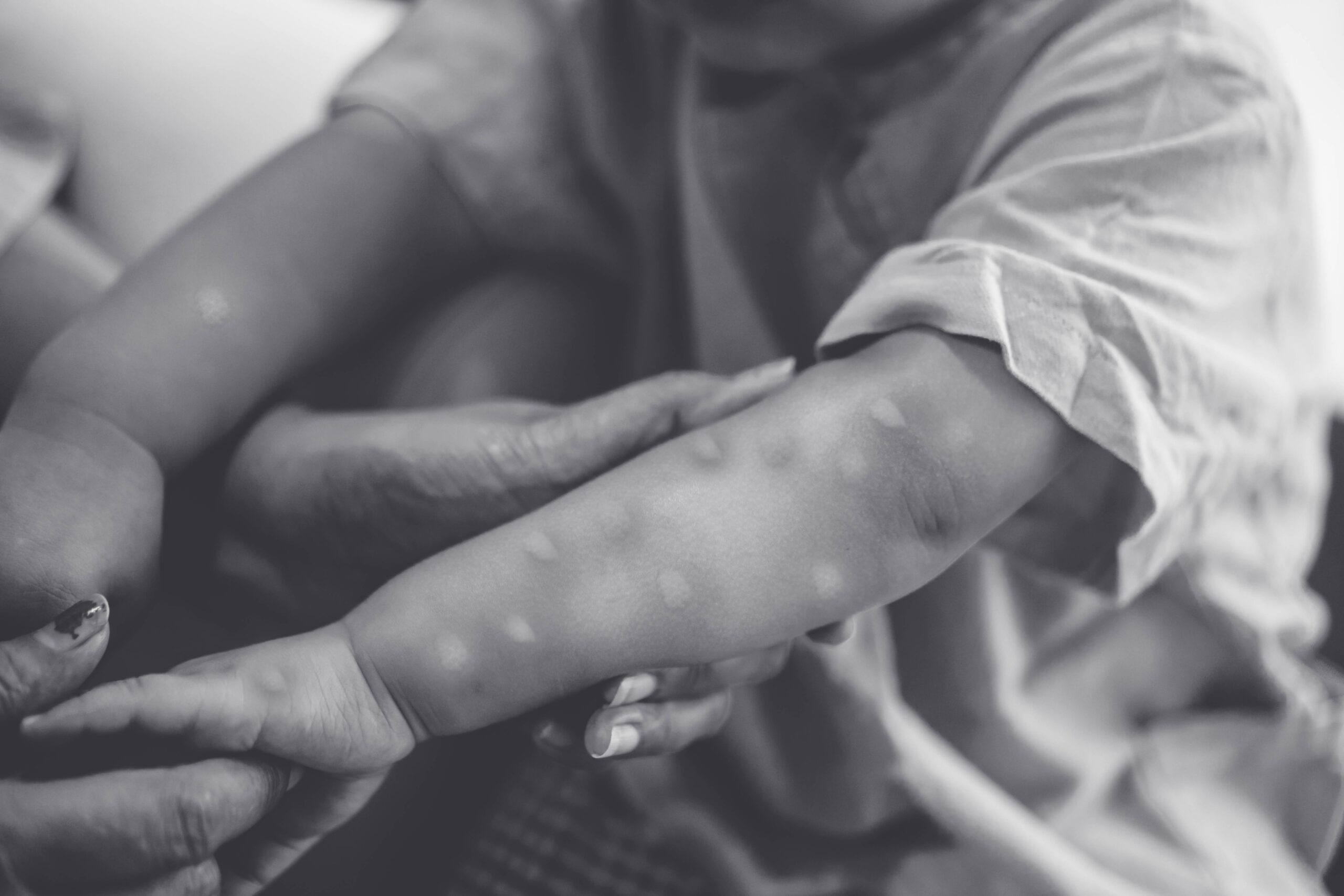
Doctors Without Borders," which continues to provide emergency medical assistance worldwide, has treated over 1,400 patients at support facilities to date. In addition to treating severe cases at general hospitals and providing outpatient care for mild cases, they are also conducting epidemiological surveillance to investigate, compile, and monitor the outbreak situation. They warn that "a comprehensive response based on community needs is necessary" to curb the spread of infections. They emphasize the need for preventive measures, as well as improvements in living conditions and care for skin lesions. Since 2022, the development of effective vaccines has been progressing.
In response to the WHO's request at the end of August, two pharmaceutical companies, one from Japan and one from Denmark, have applied for emergency use authorization for their vaccines. Director-General Tedros Adhanom stated at a press conference, "We are reviewing them promptly," suggesting that swift measures are being taken. International cooperation is steadily progressing, and we hope to monitor the situation without becoming complacent.
